
The degree to which losses to follow-up are correlated with exposure and outcome will lead to serious bias in the measures of effect of exposure and outcome. For example, individuals who develop the outcome may be less likely to continue to participate in the study. In addition, losses to follow-up may be related to the exposure, outcome or both. Cohort members may die, migrate, change jobs or refuse to continue to participate in the study. Potential sources of bias in cohort studiesĪ major source of potential bias in cohort studies is due to losses to follow-up. The failure to collect outcome data for all members of the cohort will affect the validity of study results.
COHORT STUDIES UPDATE
A great deal of cost and time is required to ensure follow-up of cohort members and to update measures of exposures and confounders, in addition to monitoring participants' health outcomes. The follow-up of study participants in a cohort study is a major challenge. Note that the method used to ascertain outcome must be identical for both exposed and unexposed groups. Outcome measures may be obtained from various sources, including routine surveillance of cancer registry data, death certificates, medical records or directly from the participant. Similarly, those in the exposed group may change their behaviour in relation to the exposure such as diet, smoking or alcohol consumption.Įxposure data may be obtained from a number of sources including medical or employment records, standardized questionnaires, interviews and by physical examination. For example, study participants may start smoking or they may fail to correctly recall past exposure. When several exposures are being considered simultaneously, the non-exposed group should comprise all those with none of the risk factors under investigation.Ī particular problem occurring in cohort studies is whether individuals in the control group are truly unexposed.
packs of cigarettes smoked per year) are measured for each individual at baseline at the beginning of the study and assessed at intervals during the period of follow-up.
COHORT STUDIES FREE
All study participants must be free of the outcome under investigation and have the potential to develop the outcome under investigation. The aim of a cohort study is to select study participants who are identical with the exception of their exposure status. In addition, information on confounding variables may be unavailable, inadequate or difficult to collect. For example, the exposure may have occurred some years previously and adequate reliable data on exposure may be unavailable or incomplete. While this type of cohort study is less time consuming and costly than a prospective cohort study, it is more susceptible to the effects of bias. In a retrospective cohort study both the exposure and outcome have already occurred at the outset of the study. A prospective cohort study is also called a concurrent cohort study, where the subjects have been followed up for a period and the outcomes of interest are recorded.

Cohort studies may be prospective or retrospective. The relative risk (incidence risk or incidence rate) is used to assess whether the exposure and disease are causally linked.

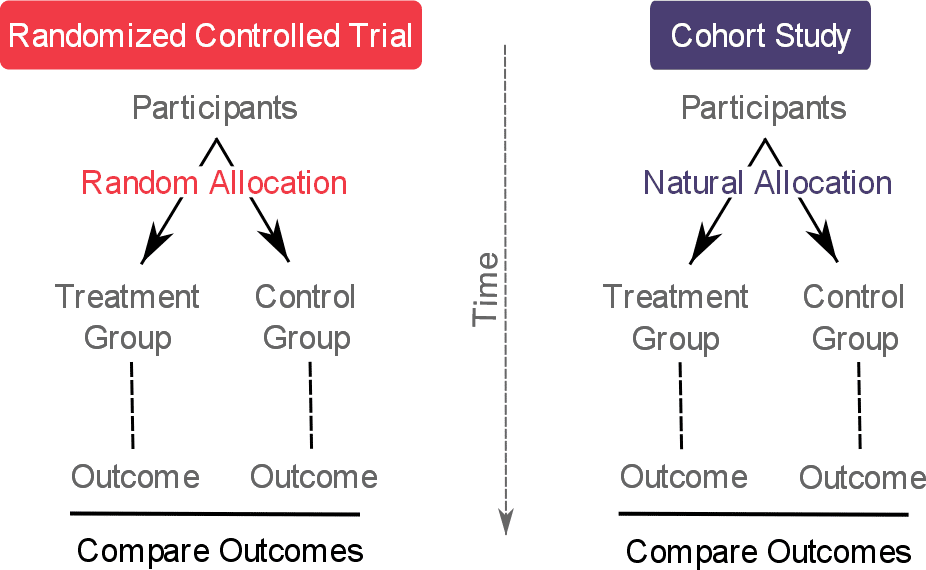
The incidence of disease in the exposed group is compared with the incidence of disease in the unexposed group. In a cohort study, a group of individuals exposed to a putative risk factor and a group who are unexposed to the risk factor are followed over time (often years) to determine the occurrence of disease. Strengths and weaknesses of cohort studies This section outlines the challenges in designing such studies, their analysis, and interpretation of outcomes.ġ. Learning objectives:You will be able to understand a cohort design, understand the differences from a case-control design, calculate the basic measures (relative risk, attributable risk etc), and appreciate its strengths and weaknesses.Ĭohort studies are a form of longitudinal study design that flows from the exposure to outcome.


 0 kommentar(er)
0 kommentar(er)
